Abstract
Background Chimeric antigen receptor (CAR) T-cell therapy has transformed treatment of relapsed/refractory hematologic malignancies, yet carries the risk of significant adverse events, namely cytokine release syndrome and neurotoxicity. In 2018, the American Society for Transplantation and Cellular Therapy (ASTCT) published a consensus grading scale to measure immune effector cell-associated neurotoxicity syndrome (ICANS). ICANS grading uses the immune effector cell encephalopathy (ICE) score to measure alterations in speech, orientation, handwriting, attention, and receptive aphasia. More subtle signs and symptoms of early neurotoxicity are not fully captured by the ICE score yet have been observed at our center and reported in the literature (Herr, Transplantation and Cellular Therapy, 2020). Thus, a more refined assessment tool that can reliably capture early neurotoxicity is needed to facilitate earlier intervention and minimize steroid use. Here, we describe the feasibility and utility of a novel neurocognitive assessment tool (CAR T-cell Neurotoxicity Screener; CART-NS) that can assess early, subtle signs of neurotoxicity after CAR T-cell therapy.
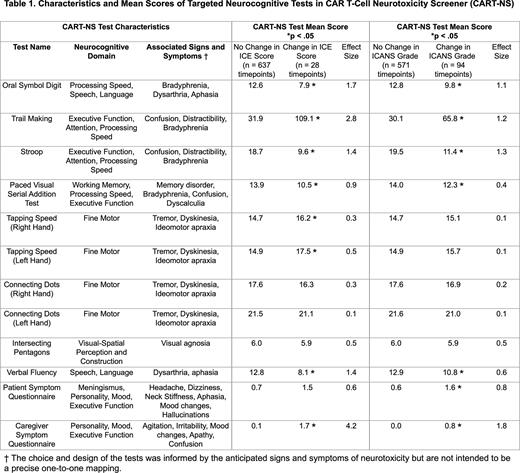
Methods We initiated a prospective, single center study examining a novel rapid neurocognitive assessment tool (CART-NS) to detect early, subtle neurotoxicity following CAR T-cell therapy. Abbreviated forms of formal neurocognitive tests were created to detect alterations in neurocognitive domains that may be seen in early ICANS. Eight tests were selected based on their ease of administration with minimal training and relatively low susceptibility to practice effects (Table 1). Symptom questionnaires were also given to CAR T-cell recipients and their caregivers to assess for new headache, dizziness, mood/personality changes, hallucinations, difficulty concentrating and irritability. During the first 14 days after CAR T-cell infusion, the resulting 10-minute battery of tests was administered every 8 hours by unit nurses, in parallel with administration of the ICE assessment. Following any change in ICE score, testing frequency was increased to every 4 hours. Outpatient CART-NS was administered daily between Day +15 and Day +30.
Results Eight patients underwent CART-NS testing during the first 30 days following CAR T-cell infusion, for a total of 665 test administrations. Patients were between 43 and 77 years old. Three patients each received axicabtagene ciloleucel and idecabtagene vicleucel, while 2 patients received tisagenlecleucel. Three patients developed Grade 2 ICANS and 2 patients developed Grade 1 ICANS. Tests within CART-NS appear sensitive in detecting early neurologic changes that correlate with ICE score changes and Grade 1 ICANS (Table 1). Changes across multiple measures and domains were observed simultaneously during Grade 1 ICANS. Among patients with changes in their ICE score (ICE < 10), the Oral Symbol Digit, Trail Making, Stroop, Paced Visual Serial Addition Test (PVSAT), Tapping Speed and Verbal Fluency tests, along with the Caregiver Symptom Questionnaire, were sensitive in detecting neurologic changes (p < .05). Among patients who developed Grade 1 or 2 ICANS, including those who did not have corresponding ICE score changes, the Oral Symbol Digit, Trail Making, Stroop, PVSAT and Verbal Fluency tests, along with Patient and Caregiver Symptom Questionnaires, were sensitive in detecting neurologic changes (p < .05). The Oral Symbol Digit, Trail Making and Stroop tests had the largest degree of change in patients who developed ICANS (Hedges’ g effect size > 0.8). Objective changes in these 3 tests were seen to precede deterioration in ICE score among patients who developed ICANS. CART-NS is easily administered by nurses, and its implementation is both feasible and reproducible.
Conclusions A subset of neurocognitive tests in CART-NS sensitively detects early neurologic and cognitive changes that correlate with alterations in ICE score and ICANS grading. Changes in these tests appear to precede deteriorations in ICE score and may have future utility in predicting impending ICANS in CAR T-cell recipients. Testing with a larger sample size across multiple centers is necessary to further validate our neurotoxicity screener. Ultimately, the systematic use of CART-NS may allow earlier intervention to minimize toxicity, mitigate the need for prolonged steroids, and optimize overall outcomes.
Disclosures
Szczepiorkowski:Fresenius Kabi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols Inc: Consultancy, Honoraria; Novartis: Consultancy; Erydel: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal